Company Profile
Company Introduction
Since its foundation in 2014, i-SENS (Jiangsu) Co., Ltd. has assembled outstanding sales and R&D teams, advanced management & control technology and automatic production lines. Based on cutting-edge biotechnology and electrochemical technology, on the road of dedication to bring people healthier lives, we are continuously keeping high quality, exploring, and making scientific and technological innovation to become a leader of POCT industry in China.
-
Product Advantages
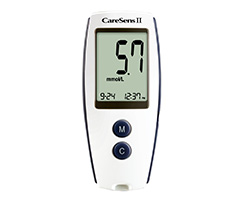
The in-hospital and out-of-hospital integrated blood glucose management system, to realize the blood glucose information management of the medical alliances, internal hospitals, internal departments and the patients.
Accuracy and anti-interference capacity complied with ISO 15197:2013
Passed external quality assessment organized by NCCL
-
Main Business
The development, sale and technical services of blood glucose management system software;
Development, consultation and service in the field of biotechnology;
R&D, manufacturing, processing, purchase and sale of medical devices (including new diagnosis reagents);
Our Vision
-

 Mission
MissionDedicated to bring people healthier lives and improve life quality by advanced healthcare technology
-

 Vision
VisionTo become a renowned POCT medical device company in China
-


 Our Values
Our ValuesIntegrity, Collaboration, Progress, Respect, Excellence
Environmental Protection
Continuous improvement and healthy development